Have you ever thought about harnessing the power of lemons? It might sound a bit zesty, but generating electricity from lemons is not only a fun experiment; it’s a fascinating dive into the world of science! With the help of tutorials available on Dailymotion, you can explore this electrifying topic and even create your own lemon battery. Let’s squeeze out the details behind this juicy phenomenon!
Understanding the Science Behind Lemon Power
So, how does a lemon generate electricity? It all boils down to the chemical reactions occurring between the components inside the fruit. Here’s a quick breakdown:
- Electrolytes: Lemons contain citric acid, which acts as an electrolyte. This means it can conduct electricity when a circuit is completed.
- Electrodes: In a typical lemon battery, you’ll use two different metals as electrodes, commonly copper and zinc. When inserted into the lemon, they create a reaction.
- Redox Reaction: The zinc electrode undergoes oxidation (loses electrons), while the copper electrode undergoes reduction (gains electrons). This flow of electrons is what generates electricity!
Here’s a simple table to visualize the components:
| Component | Function |
|---|---|
| Lemon | Provides citric acid, serving as an electrolyte |
| Copper Electrode | Acts as the positive electrode (cathode) |
| Zinc Electrode | Acts as the negative electrode (anode) |
By using these simple materials, you can create a basic battery that powers small devices like LED lights. It’s a fantastic way to combine learning with hands-on experience, making science both fun and approachable!
Also Read This: How to Change Your Behance Name
3. Materials Needed for the Experiment
Before diving into the exciting world of lemon battery experiments, let's gather all the materials you'll need. Don’t worry; most of these items are likely lying around your home!
- Fresh Lemons: You’ll need at least two medium-sized lemons. The fresher, the better, as they contain more juice and thus more electrolytes.
- Copper Coins or Copper Wire: Copper acts as one of the electrodes. If you don’t have coins, any copper wire will do!
- Zinc Nails or Galvanized Nails: These will serve as the second electrode. Zinc reacts with the lemon juice to generate electricity.
- Wires with Alligator Clips: These will connect your electrodes to a small device (like an LED or a voltmeter) to measure the electricity.
- LED Light Bulb (optional): To visually see your lemon battery in action, an LED bulb is a fun choice!
- Voltmeter (optional): If you want to measure the voltage produced, a voltmeter will come in handy.
Gather these materials, and you’ll be ready to become a lemon-powered inventor in no time!
Also Read This: Disabling Auto-Generated Chapters on YouTube for Better Control
4. Step-by-Step Guide to Producing Electricity from Lemons
Ready to make your own lemon battery? Follow these simple steps to generate electricity and impress your friends!
- Prepare the Lemons: Roll each lemon on a flat surface to soften them. This helps release more juice, increasing conductivity.
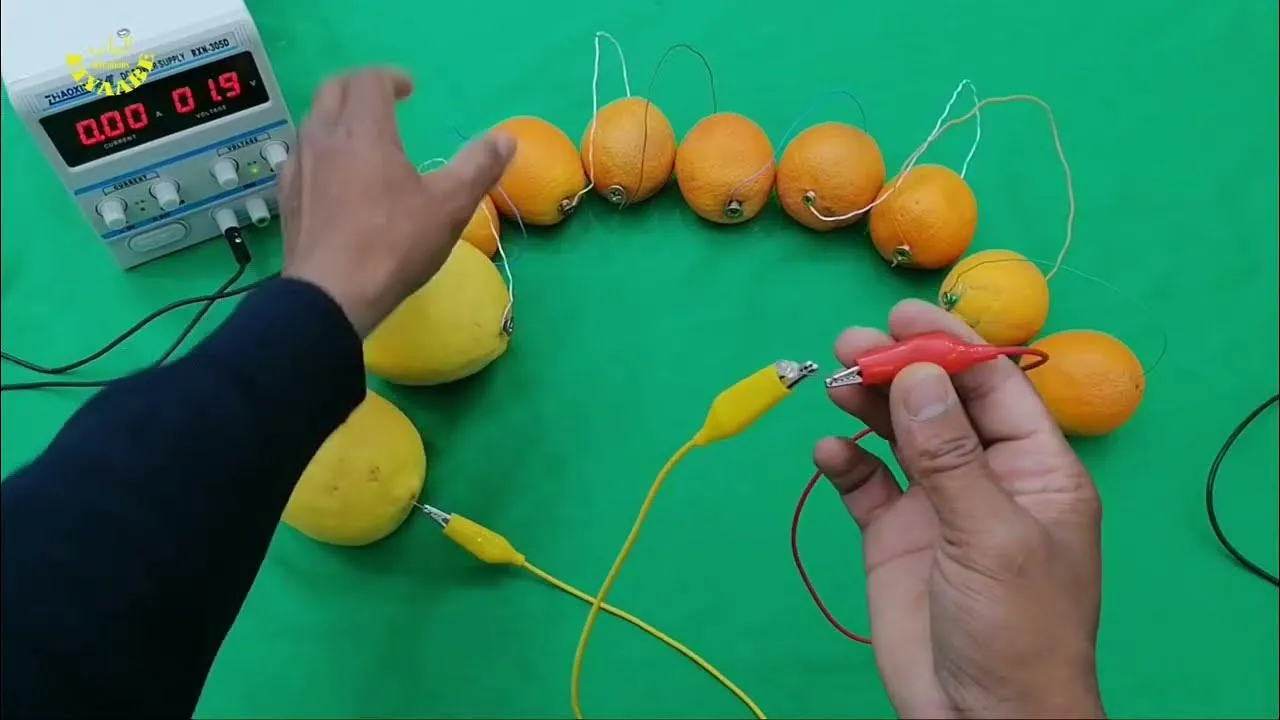
- Insert the Electrodes: Take your copper coin or wire and insert it into one end of the lemon. Then, insert the zinc nail into the other end. Make sure they don’t touch each other inside the lemon!
- Connect the Wires: Using the alligator clip wires, connect the copper electrode from the first lemon to the zinc nail of the second lemon.
- Add the LED (if using): Connect the remaining copper electrode to the positive terminal of the LED, and the free zinc terminal to the negative side.
- Observe the Results: If everything is connected properly, your LED should light up! If you’re using a voltmeter, you can measure the voltage between the electrodes.
And just like that, you’ve harnessed the power of lemons to create electricity! How cool is that?
Also Read This: How the Behance Algorithm Works
5. Exploring Dailymotion Tutorials for Visual Learning
When it comes to learning something new, especially in the realm of science experiments, visuals can make all the difference. Dailymotion offers a plethora of tutorials that guide you step-by-step through the process of generating electricity from lemons. These videos not only provide clarity but also ignite enthusiasm for DIY projects.
One of the best things about Dailymotion is the variety of content creators. You'll find everything from amateur enthusiasts to seasoned educators showcasing their experiments. This diversity means you can choose a style that resonates with you, whether it’s a fast-paced tutorial or a detailed, slow-paced explanation. Here’s what to look for in these tutorials:
- Clear Instructions: Look for videos that break down the steps clearly, showing exactly how to set up your lemon battery.
- Visual Aids: Great tutorials often include diagrams or animations that help illustrate the scientific principles at play.
- Engaging Presenters: Find creators who keep the content lively; their excitement can be contagious!
- Experiment Variations: Some videos explore different setups, using various fruits or additional components like wires and LEDs.
By following these Dailymotion tutorials, you can turn a simple kitchen experiment into an enlightening experience that combines fun with education!
Also Read This: Saving EyeEm Photos: A User’s Guide
6. Applications and Fun Facts about Lemon Power
Did you know that lemons can do more than just add flavor to your tea? They can also power small devices! The science behind this is fascinating and has various applications that extend beyond just classroom experiments. Here are some fun facts and practical applications of lemon power:
| Application | Description |
|---|---|
| Battery Technology | Lemon batteries can demonstrate principles of electrochemistry, serving as a hands-on learning tool. |
| Low-Power Devices | They can power small LEDs or clocks, showcasing how chemical reactions produce electricity. |
| Educational Tools | Teachers use lemon batteries in science classes to explain energy conversion and circuit basics. |
And here are some fun facts about lemon power:
- Lemons contain citric acid, which reacts with copper and zinc electrodes to produce electricity.
- A single lemon can produce about 0.9 volts of electricity, which is enough to light up a small LED.
- Using multiple lemons in series can increase voltage, allowing for more powerful applications.
So, the next time you slice a lemon for a drink, consider the hidden potential within! Who knew that this tangy fruit could spark such interesting science explorations?
Generating Electricity from Lemons Using Dailymotion Tutorials
Generating electricity from lemons is a fascinating and educational experiment that showcases the principles of electrochemistry. By using simple materials, you can create a lemon battery that produces a small amount of electric current. This process not only demonstrates the basic concepts of chemistry and electricity but also encourages sustainable practices by utilizing natural resources.
To create a lemon battery, you will need the following materials:
- 2 fresh lemons
- 1 copper coin or copper wire
- 1 galvanized nail (zinc-coated)
- 2 insulated copper wires with alligator clips
- 1 small LED light or a digital multimeter
The steps to generate electricity from lemons are straightforward:
- Roll the lemons on a hard surface to soften them.
- Insert one copper coin into one lemon and one galvanized nail into the other lemon.
- Connect the copper coin from the first lemon to the galvanized nail of the second lemon using one copper wire.
- Connect the free end of the copper wire to the positive terminal of the LED.
- Connect the other free end of the LED to the copper coin using the second copper wire.
Once everything is connected, the LED should light up, demonstrating that electricity can be generated from lemons. Dailymotion hosts numerous tutorials that provide visual guidance on setting up your lemon battery, making it easier to follow along and understand the science behind the experiment.
In conclusion, generating electricity from lemons is an engaging way to learn about renewable energy and the principles of electrochemistry. Utilizing Dailymotion tutorials can enhance your understanding and make the process more accessible, allowing anyone to explore the wonders of science in a fun and interactive manner.